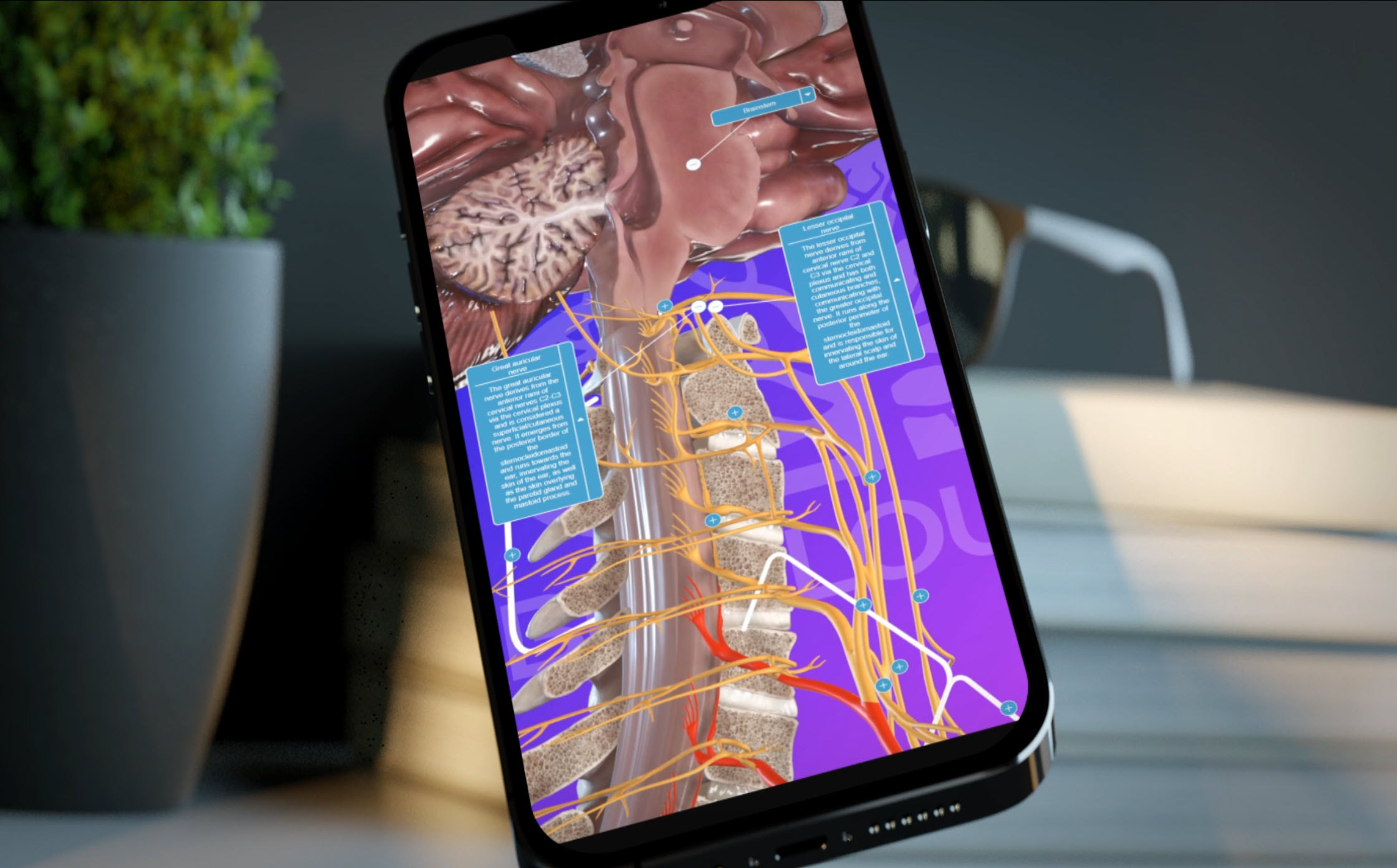
Why AI-Generated Content Is Not Appropriate for Medical Animation in Pharma and Biotech
In regulated medical communications, even minor inaccuracies can lead to misinterpretation by healthcare professionals, increased regulatory scrutiny, and potential compliance violations.
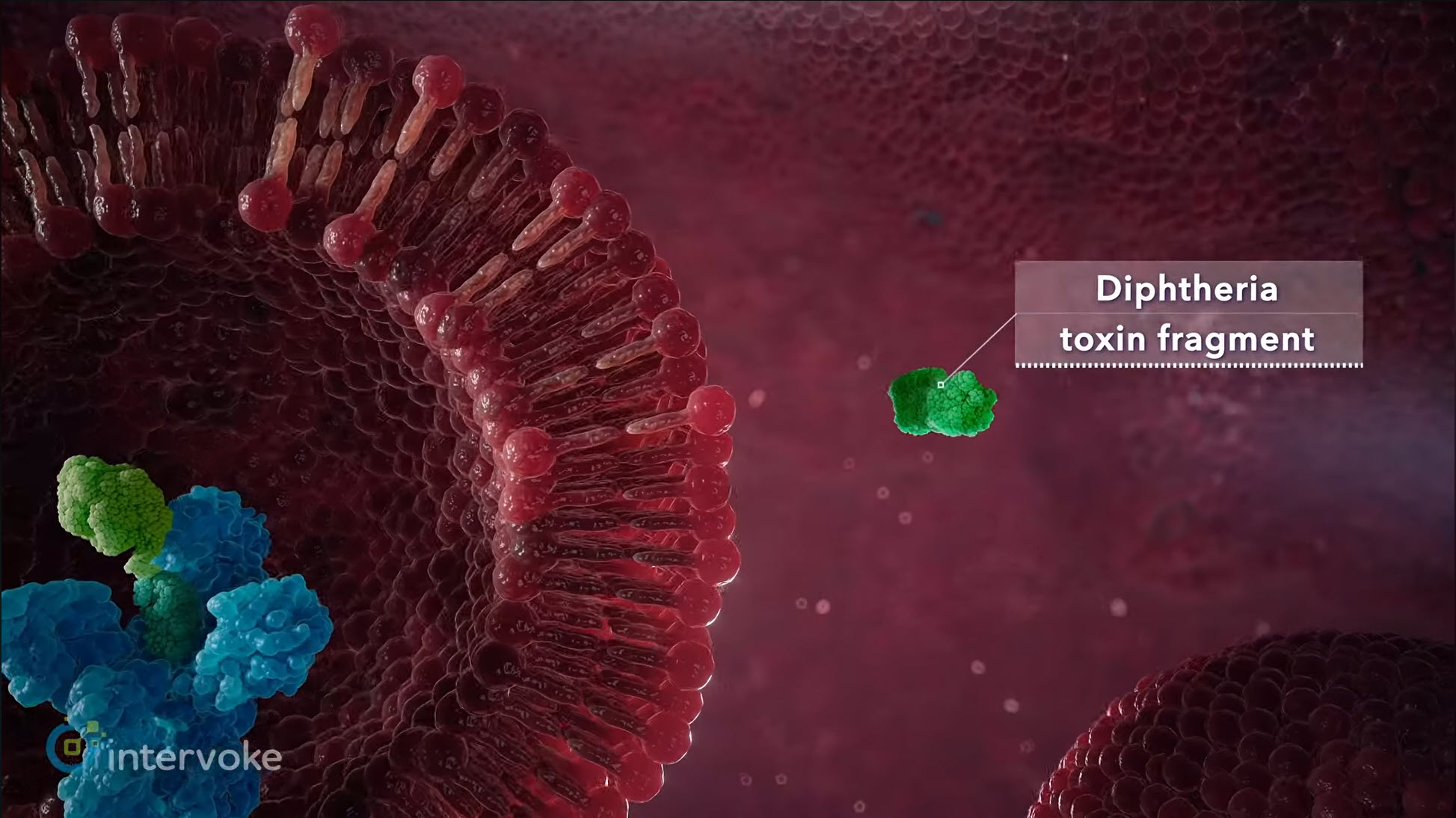
As artificial intelligence gains visibility across creative industries, questions have emerged regarding its applicability to medical animation and scientific visualization. While AI may have limited utility in consumer or entertainment content, its use in regulated life-science communications presents substantial scientific, legal, and operational risks.
For pharmaceutical, biotech, and medical device organizations, medical animation is not decorative content—it is regulated scientific communication. As such, AI-generated animation is fundamentally incompatible with the requirements of medical accuracy, intellectual property protection, regulatory compliance, confidentiality, and production flexibility.
Medical Accuracy Cannot Be Automated
Medical animation requires precise interpretation of complex biological systems, therapeutic mechanisms of action, and medical device functionality. These visuals are frequently based on proprietary data, unpublished research, internal clinical insights, and evolving scientific understanding.
AI systems do not interpret or validate science. They generate outputs based on probabilistic pattern recognition rather than biomedical reasoning. This creates unacceptable risk, including:
- Anatomical and physiological inaccuracies
- Misrepresentation of molecular or cellular mechanisms
- Incorrect device behavior or procedural workflows
- Visual oversimplification that alters scientific meaning
In regulated life-science communications, even subtle inaccuracies can compromise scientific credibility and trigger compliance concerns.
Therapeutics and Devices Do Not Exist in Public Data Sets
Unlike consumer products or general anatomy, pharmaceutical therapeutics and medical devices are often novel, proprietary, and absent from public data sets.
AI models are trained on existing content. They cannot accurately visualize investigational drugs, new modalities, proprietary delivery systems, or custom devices because no authoritative reference material exists in the public domain. Any AI-generated depiction in these cases is, by definition, speculative.
Fabrication or extrapolation of scientific content is unacceptable in medical animation, where traceability, source validation, and scientific accountability are required.
AI-Generated Video Is Not Editable—And That Breaks the Medical Animation Workflow
A critical but often overlooked limitation of AI-generated animation is that once a 3D model has been incorporated into an AI system and rendered into a video, the output is effectively static. The underlying model is no longer accessible as a manipulable asset.
This directly conflicts with how professional 3D animation works—across any genre, but especially in life sciences.
In traditional 3D animation, the creation of a scientifically accurate 3D model is only the beginning. That model is intentionally built to be reusable, adaptable, and editable. It can be re-posed, re-lit, re-sequenced, and revised repeatedly to accommodate feedback, new data, or regulatory requirements.
In pharma and biotech animation, extensive changes are not the exception—they are the norm. As content progresses through internal scientific review, medical-legal review, and regulatory approval, multiple iterations are required. These may include:
- Adjustments to mechanisms of action
- Changes to anatomical emphasis
- Refinement of biological timing or sequencing
- Removal or modification of claims-adjacent visuals
- Updates driven by evolving clinical data
AI-generated video does not support this iterative process. Once rendered, the content cannot be meaningfully revised without regenerating the entire video—often introducing new inaccuracies or inconsistencies. This makes AI-generated animation operationally useless in regulated medical workflows.
Professional 3D animation exists precisely because it allows for controlled, traceable, and compliant revision. AI output eliminates that control.
Intellectual Property and Copyright Exposure
AI-generated visuals introduce significant intellectual property risk. Because AI models are trained on large, uncurated data sets, there is no reliable mechanism to ensure that outputs are original, non-derivative, or free from embedded third-party intellectual property.
This creates exposure to:
- Copyright infringement claims
- Trade secret contamination
- Loss of exclusivity or competitive advantage
- Legal disputes over content ownership
Pharmaceutical and biotech companies invest heavily in proprietary science and brand differentiation. Recycled or derivative visuals—whether intentional or inadvertent—undermine that investment and introduce unnecessary legal risk.
Regulatory and Medical-Legal Compliance Risks
Medical animation used in pharma and biotech marketing, education, or investor communications must align with approved labeling, substantiated claims, and internal medical-legal review standards.
AI-generated animation cannot reliably:
- Distinguish on-label from off-label content
- Apply jurisdiction-specific regulatory requirements
- Maintain audit trails for claims substantiation
- Respond to nuanced medical-legal feedback
Without clear authorship, documentation, and expert accountability, AI-generated visuals are extremely difficult to defend during regulatory review.
Confidentiality and Non-Disclosure Obligations
Pharmaceutical and medical device development is governed by strict confidentiality and non-disclosure agreements. Scientific data, mechanisms, and design specifications are shared under legal obligation and must remain secure.
The use of AI systems—particularly cloud-based or third-party models—poses inherent risks related to data ingestion, retention, and reuse. Even the perception that confidential information could be incorporated into a generative system is unacceptable to most legal and compliance teams.
For this reason alone, AI-generated medical animation is incompatible with NDA-protected workflows.
Pharma Does Not Want Recycled Animation
Pharmaceutical companies require bespoke, scientifically precise animation tailored to their specific therapeutic, device, and messaging strategy.
Medical animation must be:
- Purpose-built
- Scientifically validated
- Legally defensible
- Exclusively owned
AI-generated content cannot guarantee originality, exclusivity, editability, or long-term usability.
The Role of Experts in Medical Animation
Medical animation remains a discipline that demands human expertise—scientific literacy, regulatory awareness, and strategic judgment. While automation may support limited technical tasks, the core work of scientific visualization must remain under expert control.
At Intervoke, we develop medical animation through rigorous scientific review, iterative collaboration, and strict adherence to regulatory and legal standards. Our 3D assets are designed to evolve alongside the science, withstand medical-legal scrutiny, and remain fully editable throughout the lifecycle of a program.
In regulated life-science communications, accuracy, control, and compliance are not optional—and they cannot be automated.
Set up a Discovery Call to learn more about Intervoke’s approach to compliant, high-fidelity medical animation and interactive 3D anatomy.
Let’s Get Started
Interested in learning more about how Intervoke can bring your science to life?
Contact us or book a Discovery Call to get started.